 |
| (c) OMICS |
This study has been published in the journal JAMA Ophthalmology.
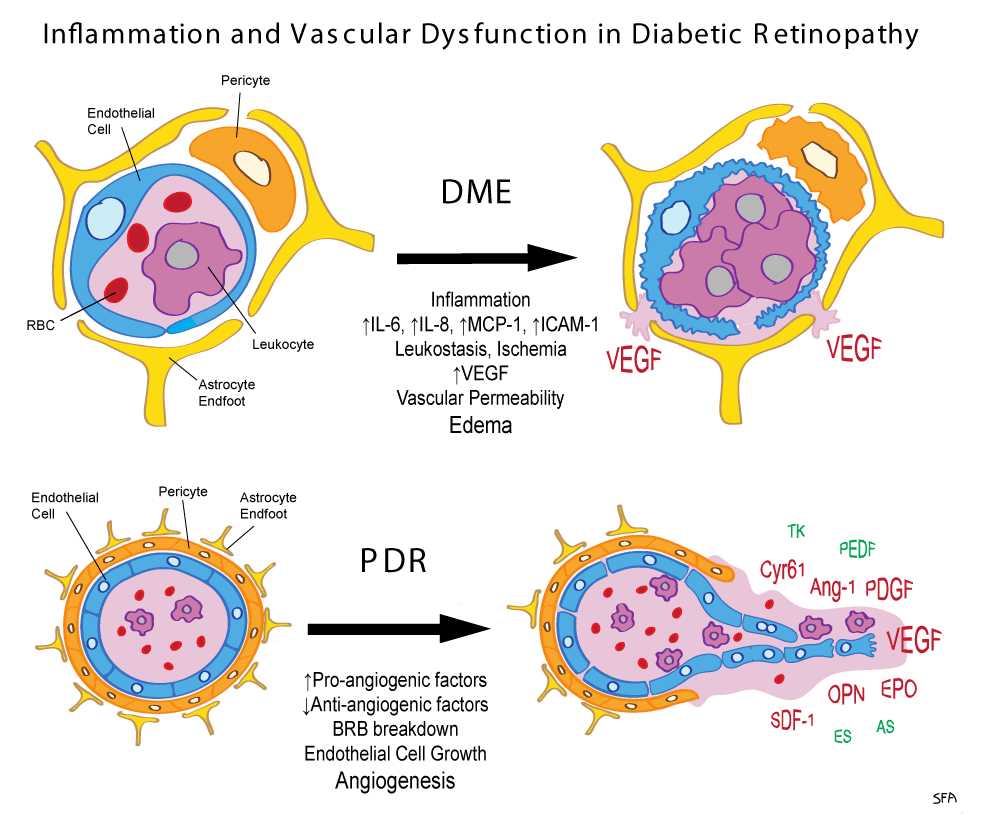
Diabetic macular edema is the accumulation of fluid in the macula, an area in the centre of the retina responsible for sharp, straight-ahead vision. The fluid buildup causes the macula to swell and thicken, which blurs vision. Dr. Muni said it is the leading cause of vision loss in people under the age of 65 in North America.
Treatment for diabetic macular edema has evolved dramatically in the last decade, but still requires a sometimes painful injection in the eye, in some cases as often as once a month.
In this study, the research involved collecting fluid samples from the front, or anterior, chamber of the eyes of 48 patients who were then treated with a ranibizumab injection (LucentisTM).
They found there were two molecules that were associated with who responded better to the injections than others. The first, which was somewhat expected, was low levels of vascular endothelial growth factor, or VEGF, a protein produced in the eye when there is poor blood flow. Ranibizumab is known to bind to VEGF, reducing the amount of VEGF in the eye, meaning there is less leakage from blood vessels and less fluid accumulation.
But the biggest predictor of who will respond to treatment, as per the authors, was high levels of a different molecule, Intercellular Adhesion Molecule 1, referred to as ICAM-1, one of a group of molecules known as cytokines that both help cells communicate immune responses and stimulate the movement of cells toward sites of inflammation.
As per Dr. Muni, who was responsible for this study: "The prospect of ongoing injections in the eye is daunting for patients. The fact that we can now measure a protein in the eye that allows us to predict which patients are less likely respond to treatment, could lead to more personalized and tailored medicine and fewer injections. This could alleviate the treatment burden on patients and the health-care system.”
St. Michael’s Hospital, where this study was conducted, is enrolling people for a bigger followup trial to determine the optimal interval between injection treatments based on the levels of these biomarkers.
The study received financial support from Novartis Pharmaceuticals Canada, Inc., manufacturers of LucentisTM, and the Retina Foundation of Canada.
Sources: 1 2
More about Retina Global here. We seek your support. Click here to donate.
No comments:
Post a Comment
Thanks for your comments. We will get back to you shortly if there is a need to respond to it.
- Admin, Retina Global
Read more on Retina Global.